Regulatory submissions, simplified. author once. submit globally.
Dossian is the regulatory platform for medical devices. Author your submission content once, then generate jurisdiction-specific dossiers for FDA 510(k), EU MDR, and beyond.
Jane Doe
jane@acme.com
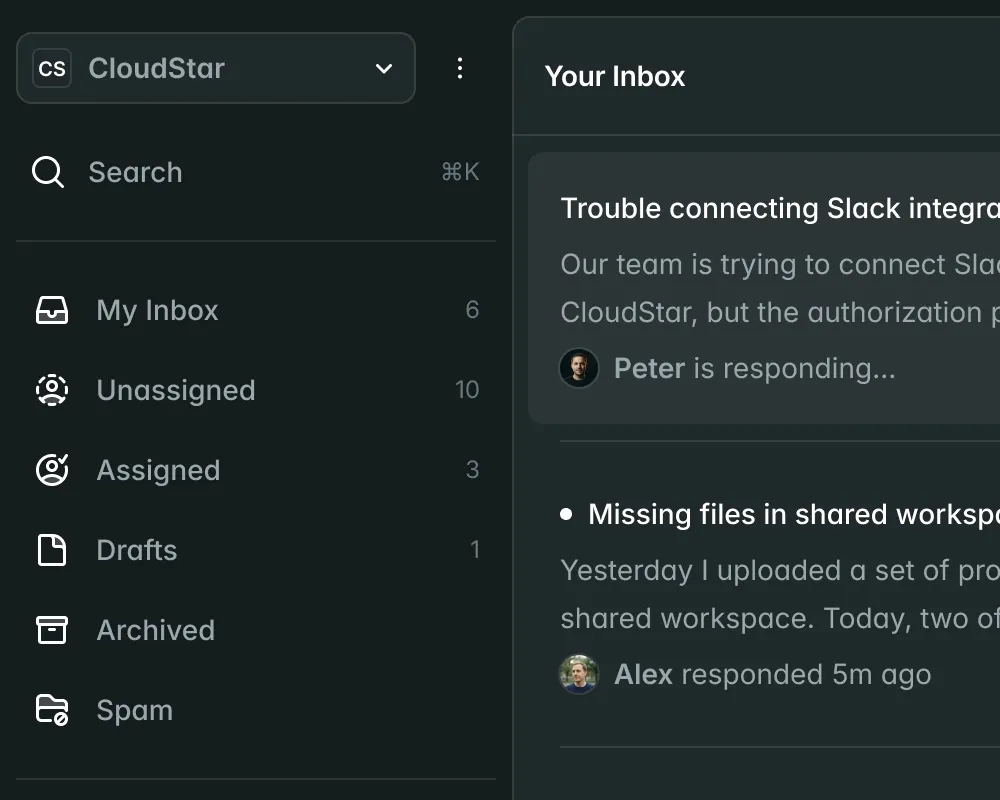
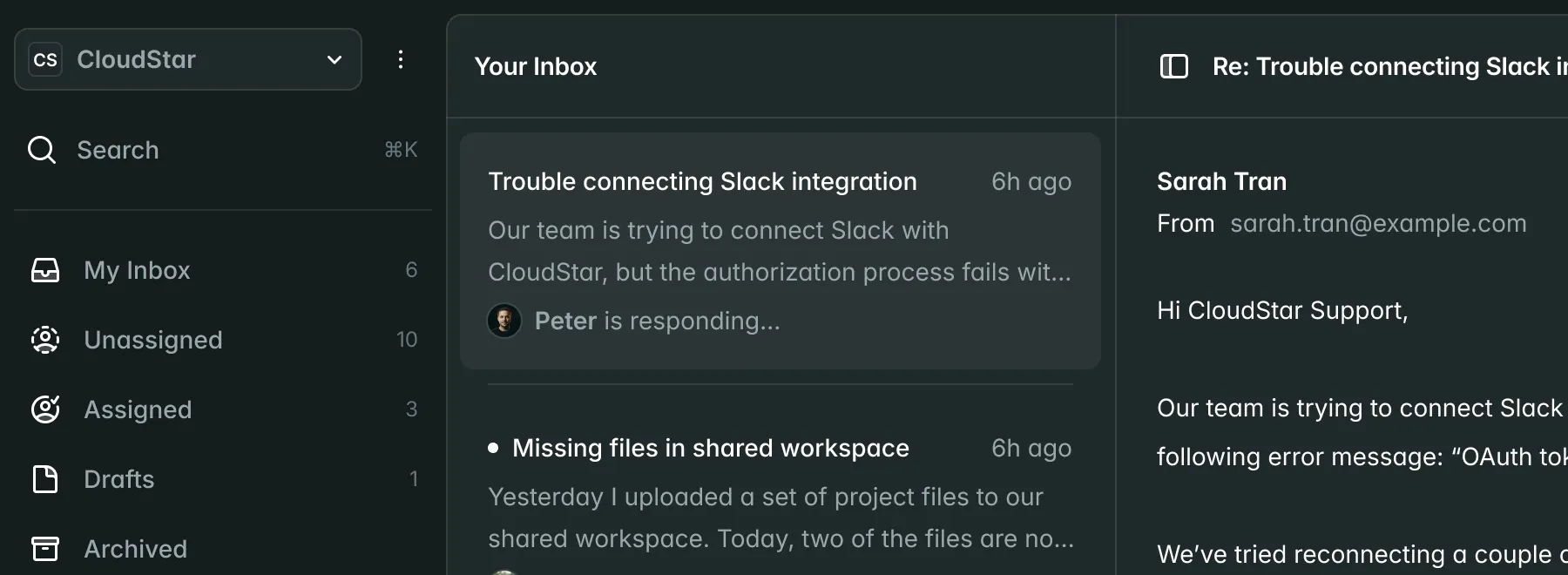
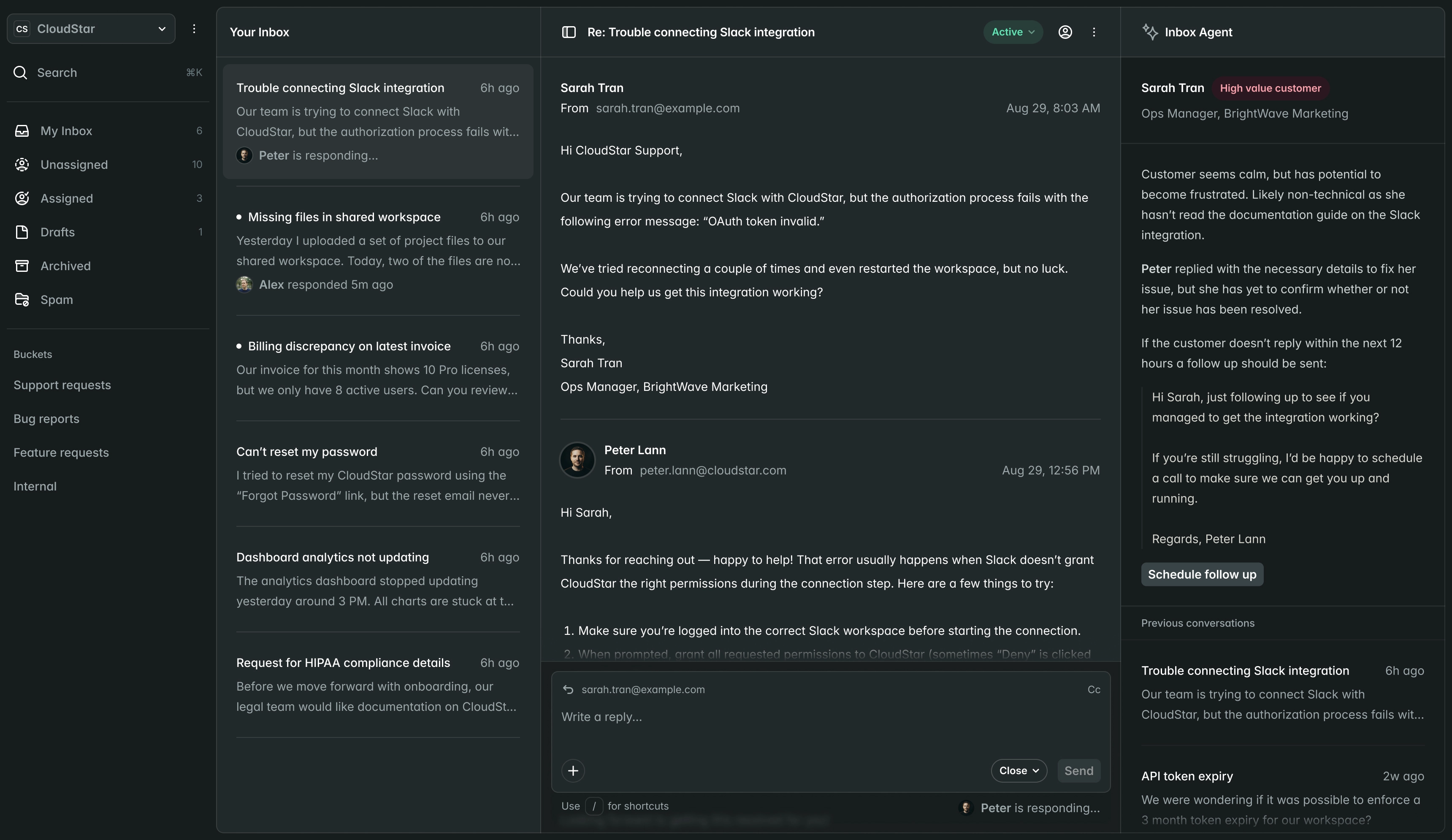
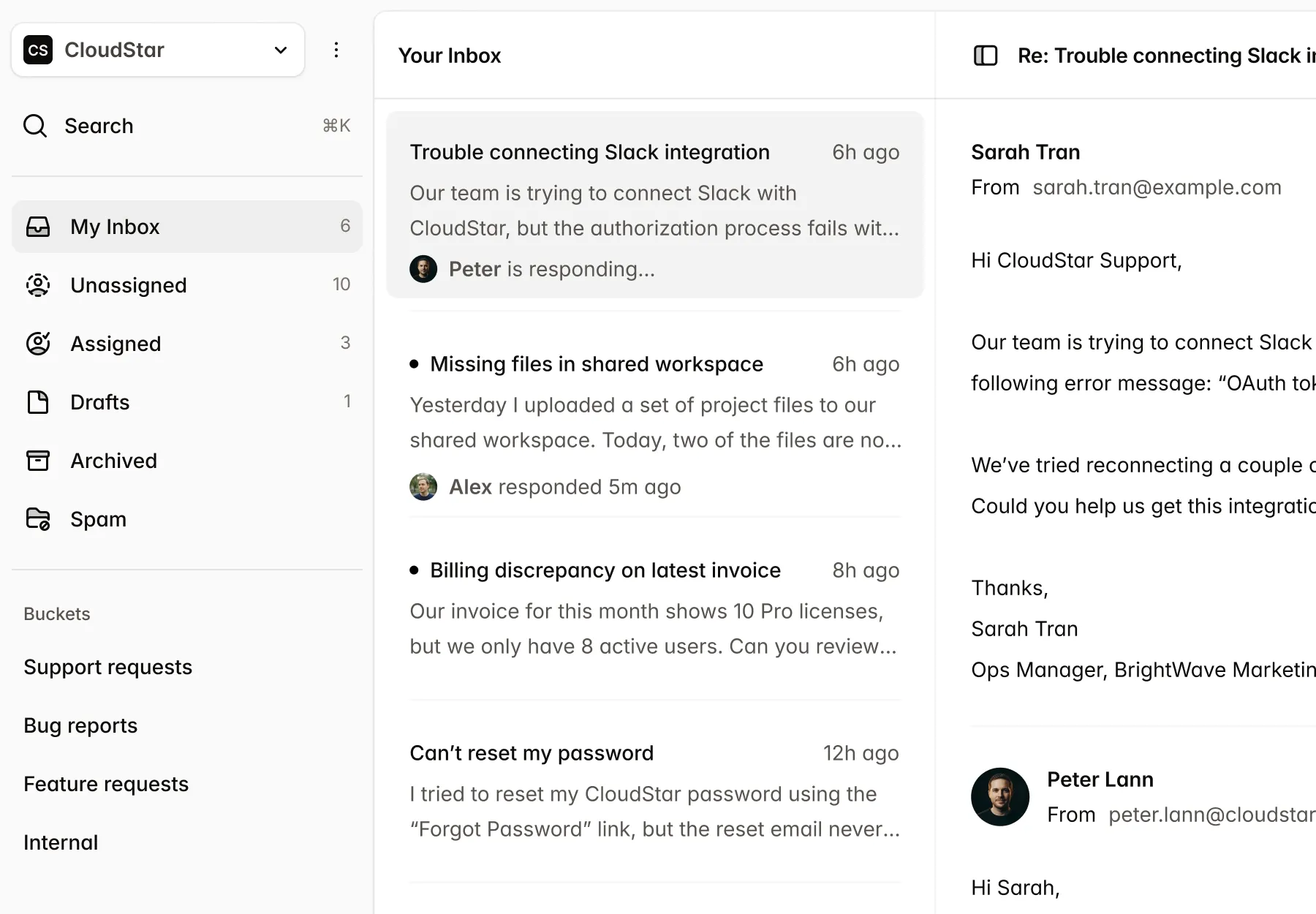
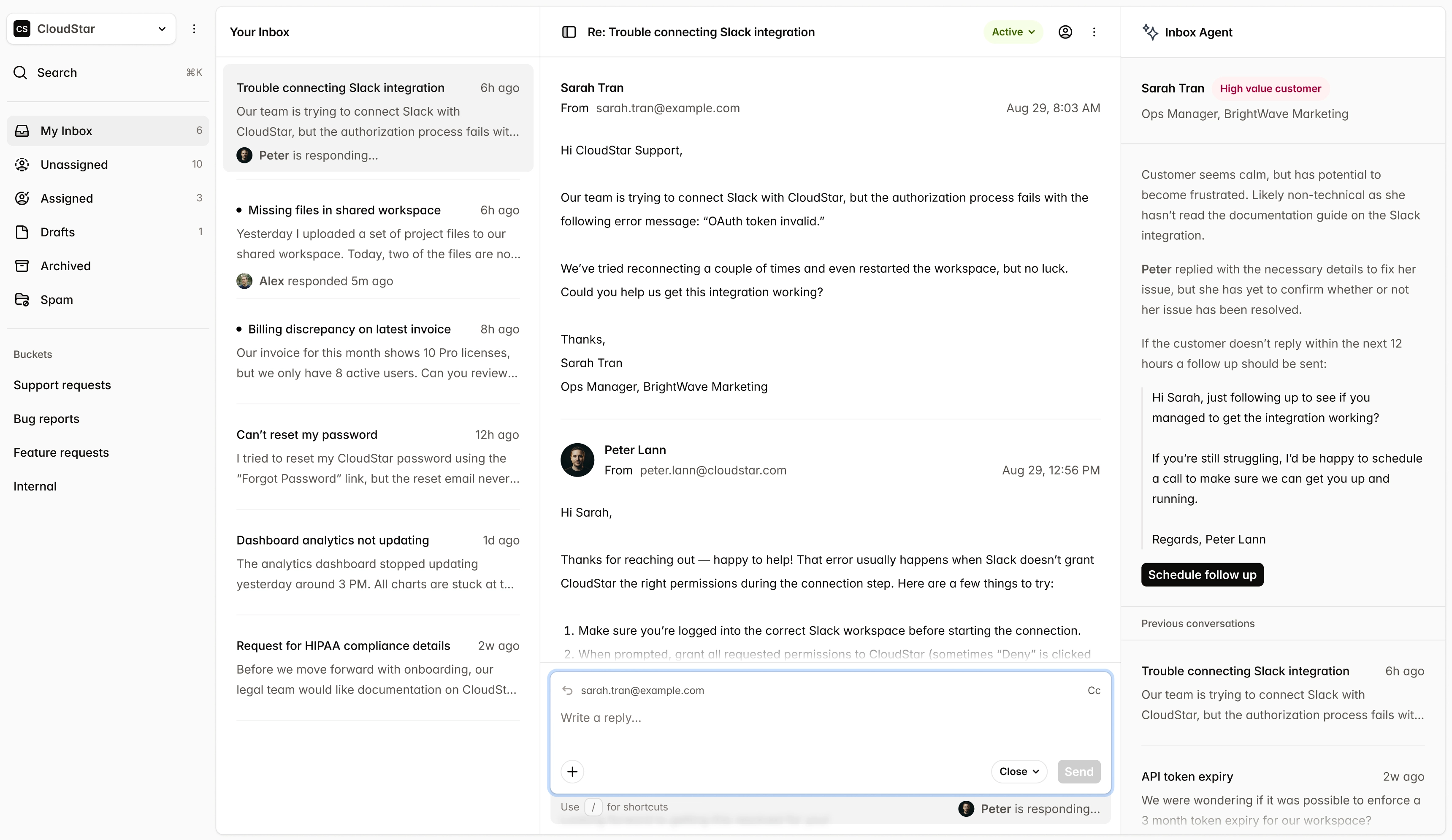
Submissions
New Submission| Name | Market | Status | Updated |
|---|---|---|---|
CardioFlow Monitor 510(k) | US | Submitted | Jan 15 |
NeuroPulse Stimulator PMA | US | In Review | Jan 12 |
OrthoFlex Joint System EU MDR | EU | Draft | Jan 10 |
DermaVision Scanner 510(k) | US | Approved | Dec 28 |
PulmoTrack Analyzer EU MDR | EU | Action Required | Dec 20 |
Jane Doe
jane@acme.com
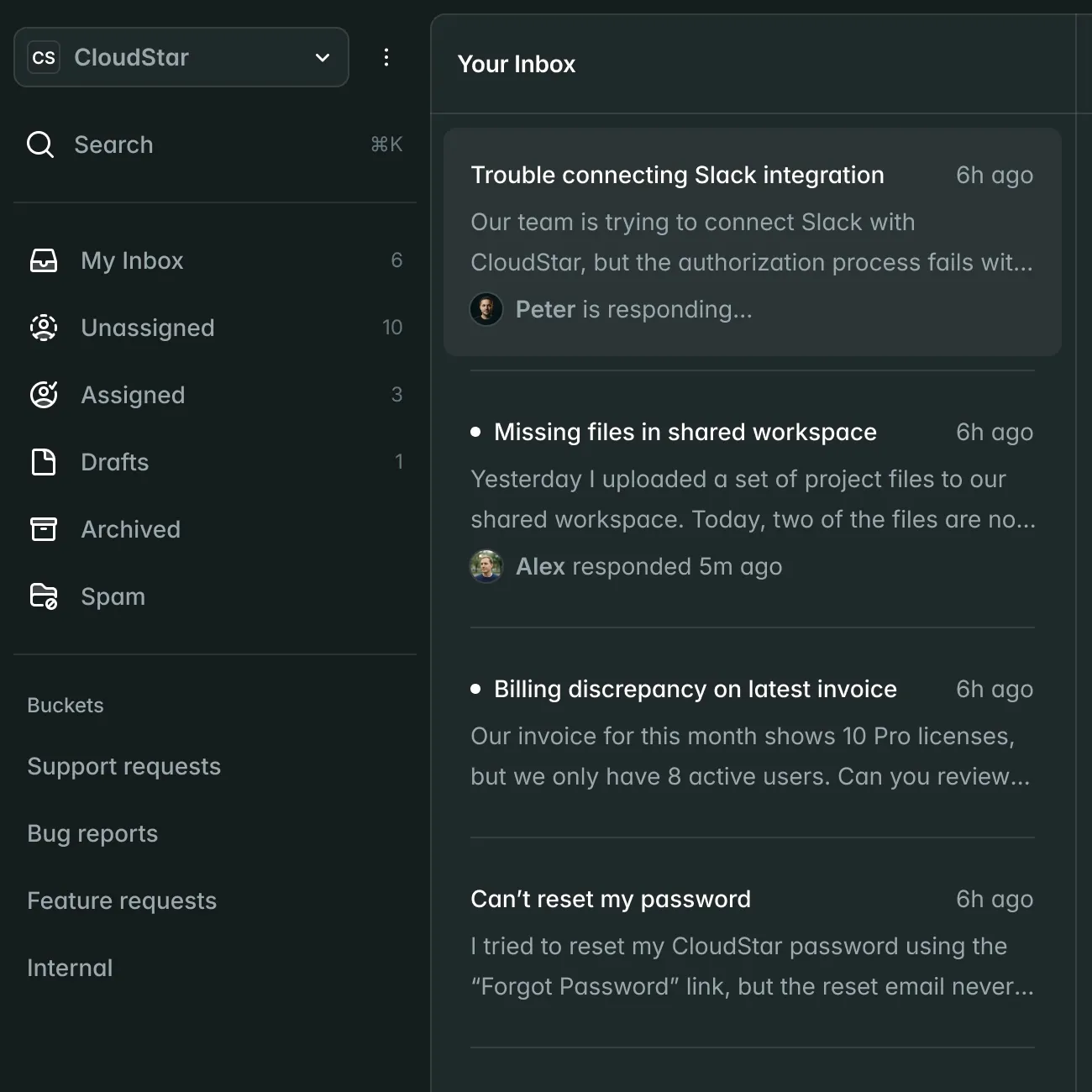
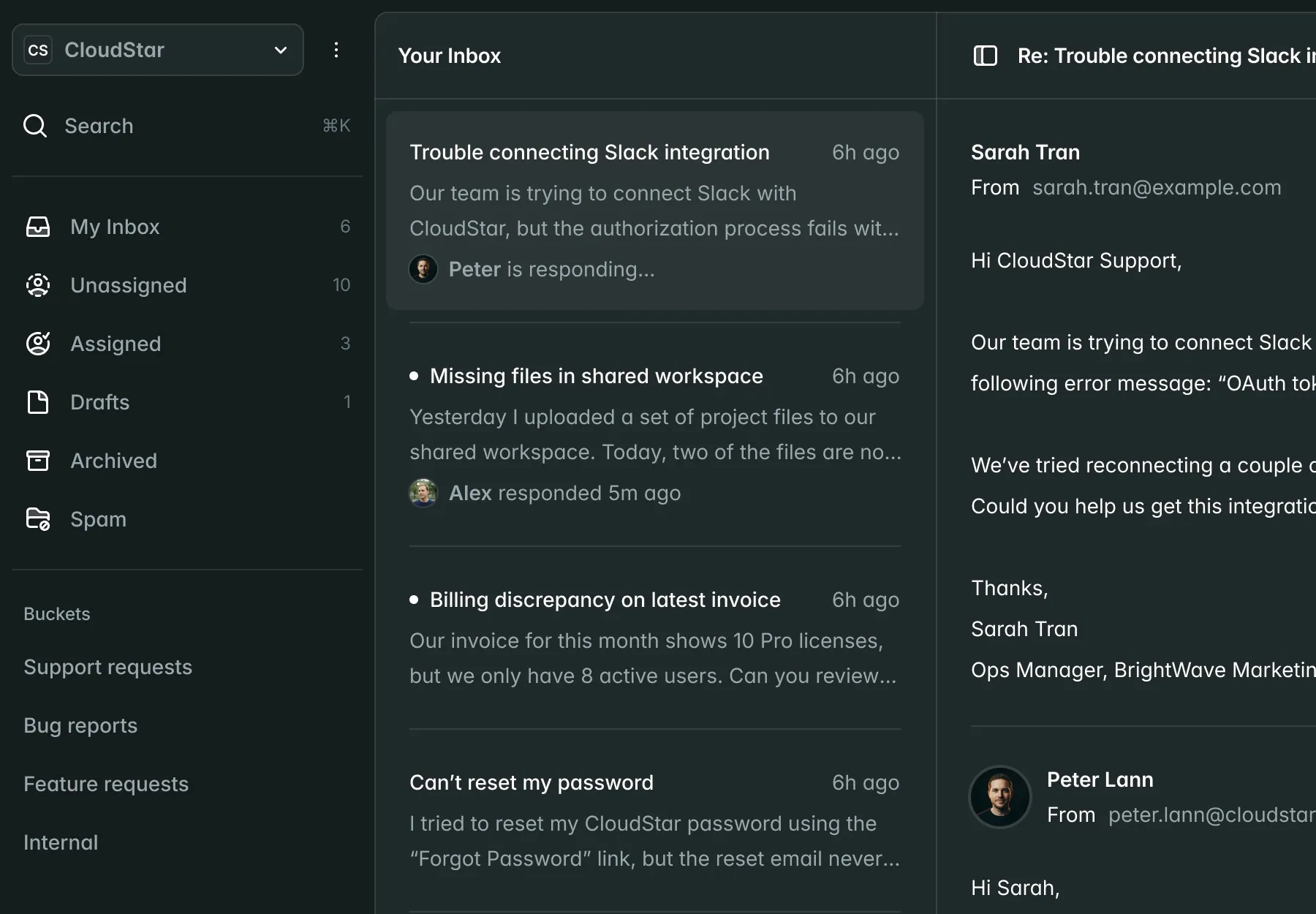
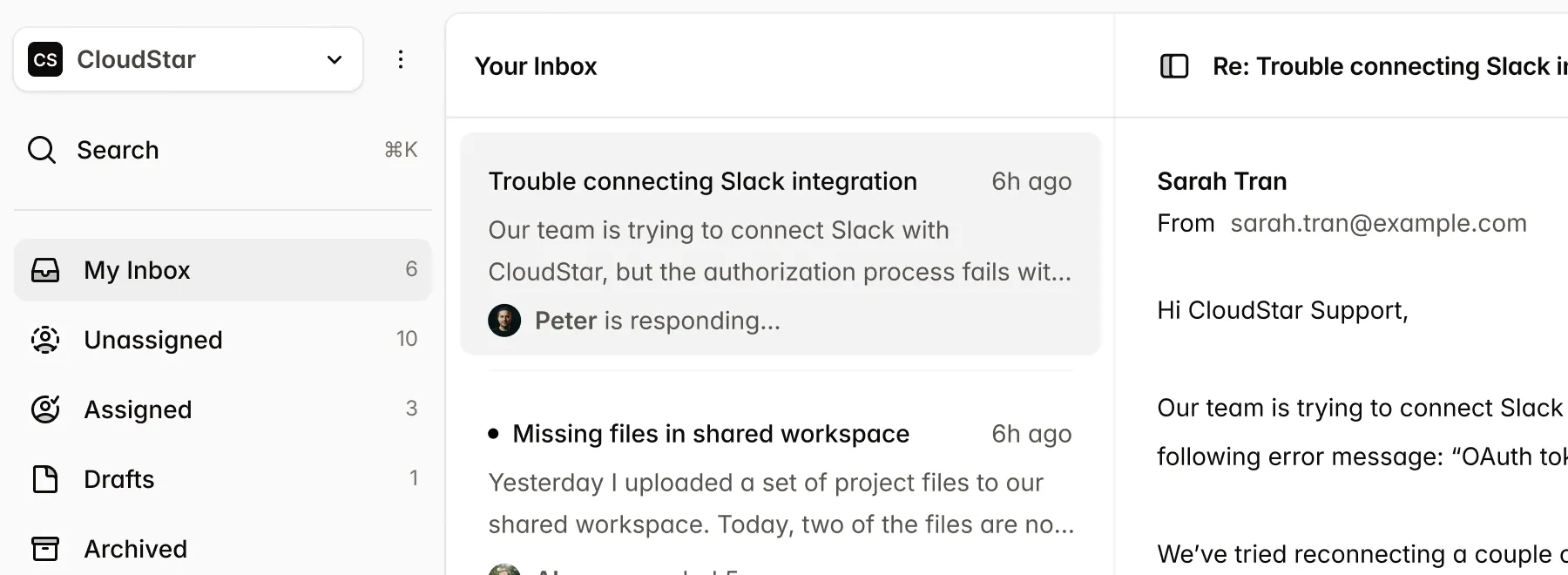
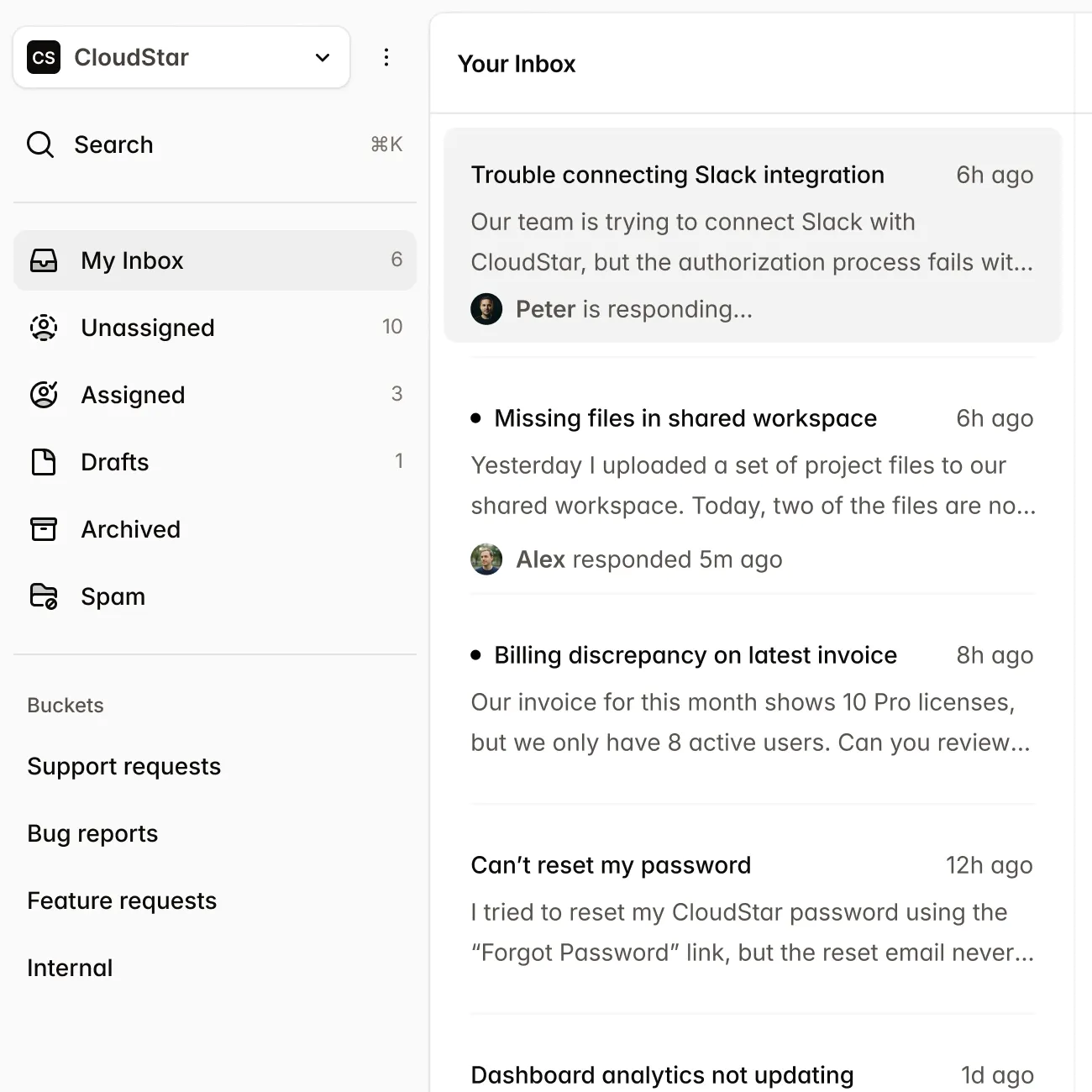
Submissions
New Submission| Name | Market | Status | Updated |
|---|---|---|---|
CardioFlow Monitor 510(k) | US | Submitted | Jan 15 |
NeuroPulse Stimulator PMA | US | In Review | Jan 12 |
OrthoFlex Joint System EU MDR | EU | Draft | Jan 10 |
DermaVision Scanner 510(k) | US | Approved | Dec 28 |
PulmoTrack Analyzer EU MDR | EU | Action Required | Dec 20 |
Everything you need to bring medical devices to market faster
Work smarter, move faster, and keep every submission details right where it belongs — in IMDRF ToC.
One click e-STAR submission generation
Auto-generate compliant 510(k), PMA, and De Novo submissions. Built-in validation against CDRH requirements, with section-by-section guidance.
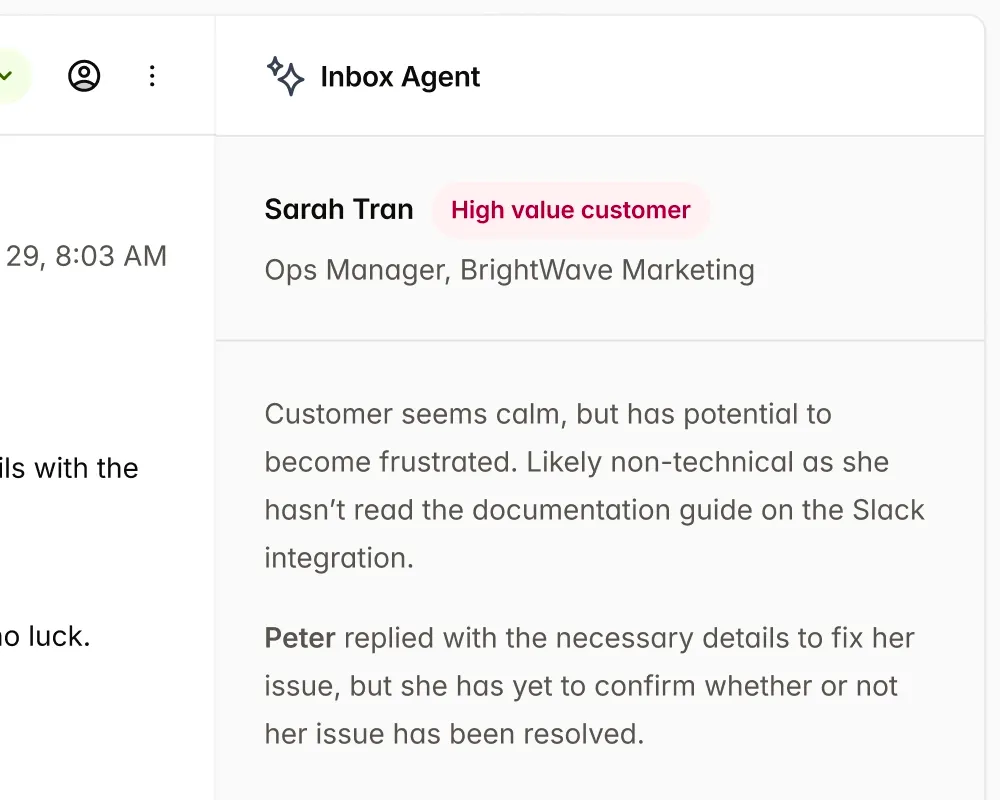
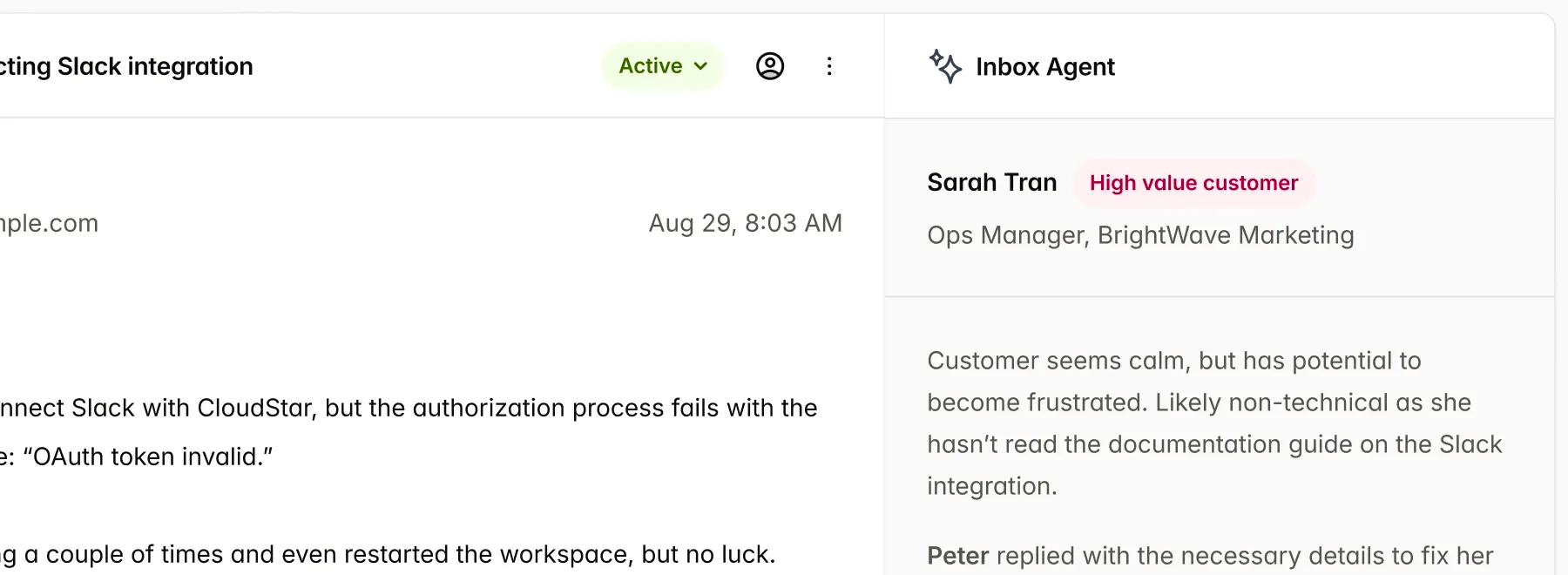
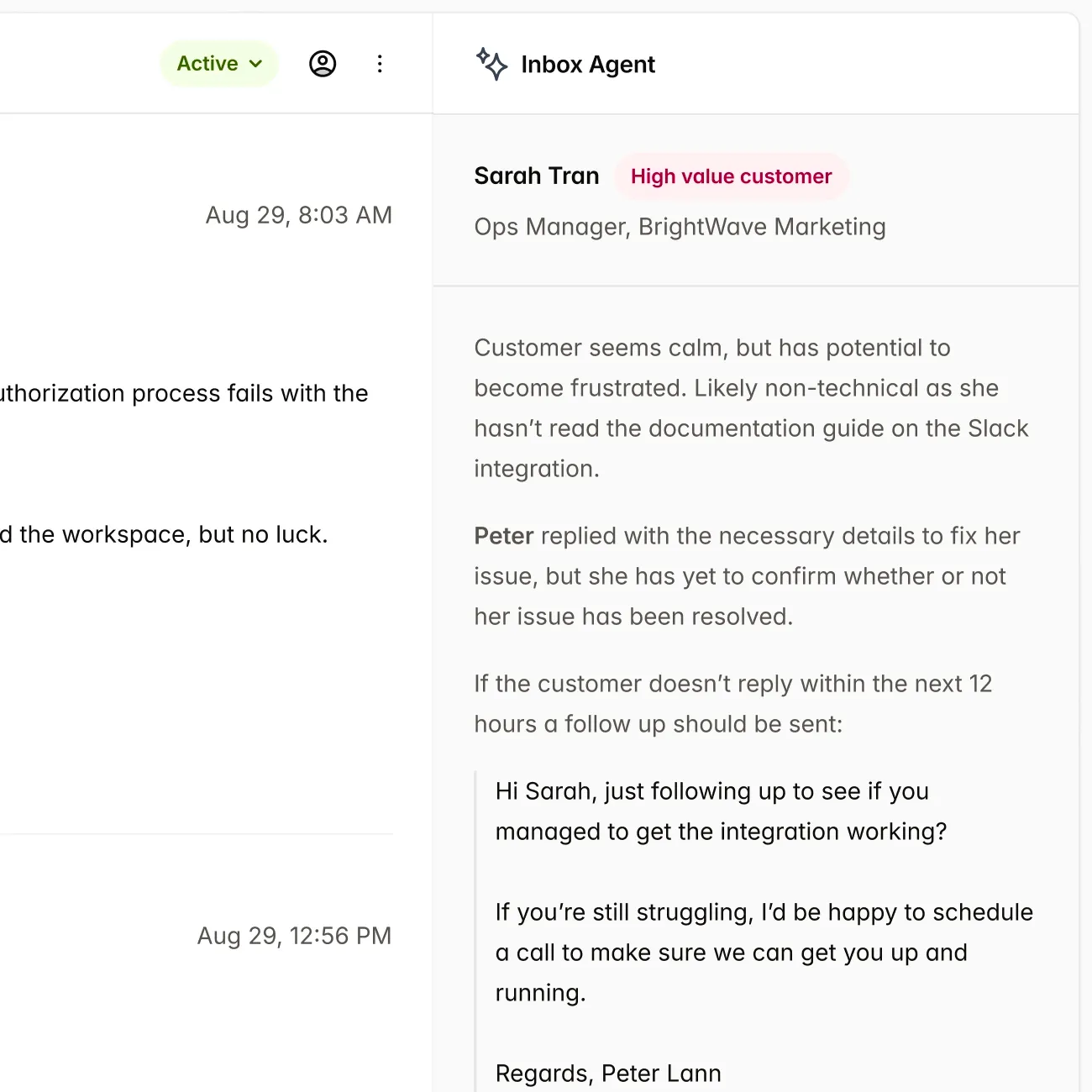
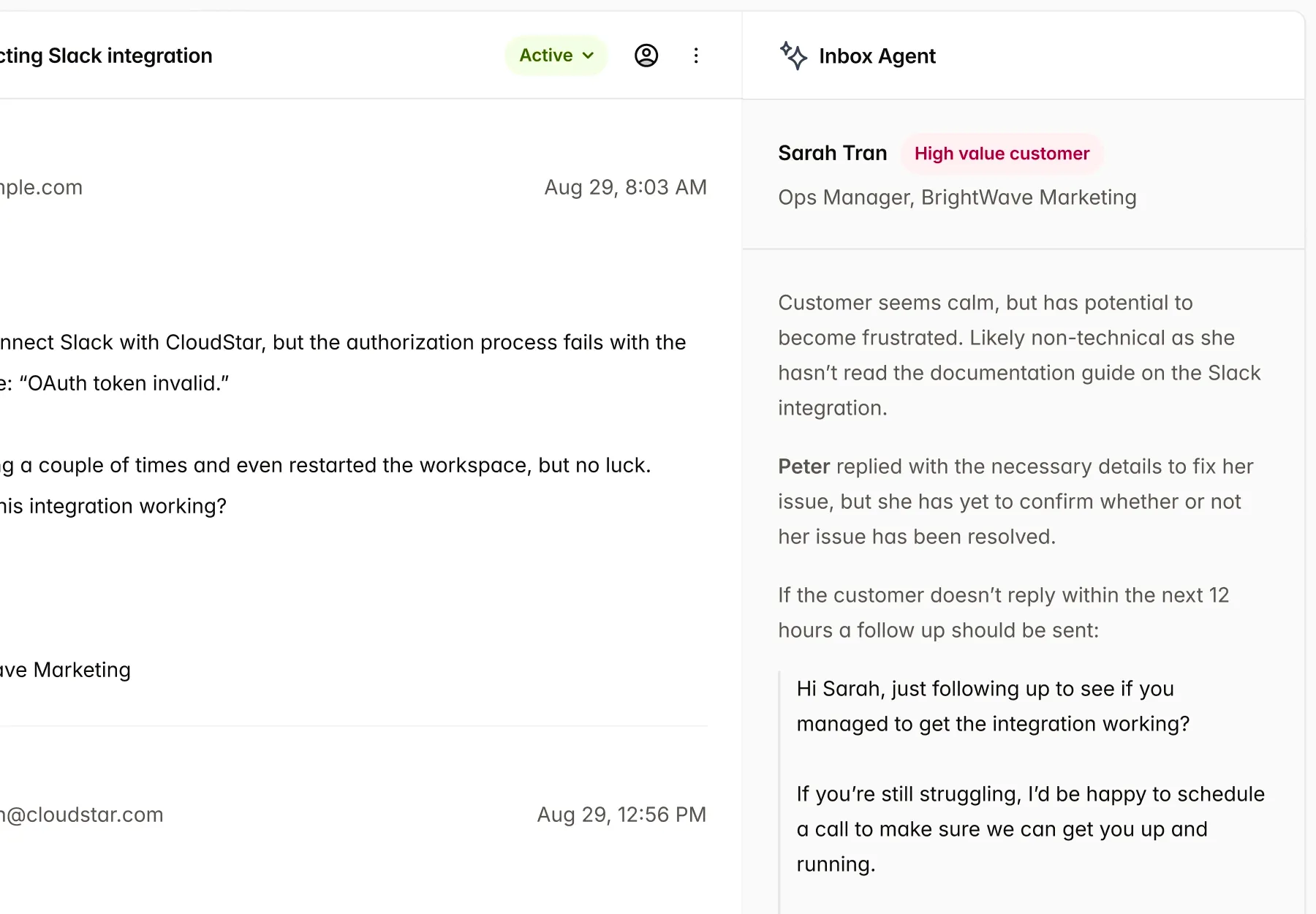
Single source of truth, for all Market
IMDRF ToC native content slots allows to use content reuse across market, with support for region specific templates.








Submission Collaboration
Securely work with consultants, clients, and regional partners on shared submissions, with comments, version history and audit logs.




Manage Global Registration
Manage product registration and certificates lifecycle by country. Stay updated on upcoming expirations and renewals with automated alerts.




Submission project management
Assign tasks to internal or external (consultants, representative) team member. Automated dependency tracking and submission-ready checklists.
Everything you need to bring medical devices to market faster
Work smarter, move faster, and keep every submission details right where it belongs — in IMDRF ToC.
Regulatory pathway modeling
AI-guided workflow, helps with product classification, risk profile and a tests & standards plan.
Human in the loop
Our AI agents asks clarifying questions to remove ambiguity from any workflow.
Predicate analysis in seconds
AI agent finds similar devices instantly, compare key characteristics with confidence score and reasoning.
Questions & Answers
Ready to simplify your next regulatory submission?
Author your regulatory content once, generate submission-ready dossiers for every market, and let AI-powered tools guide you through predicate search, classification, and pathway strategy.